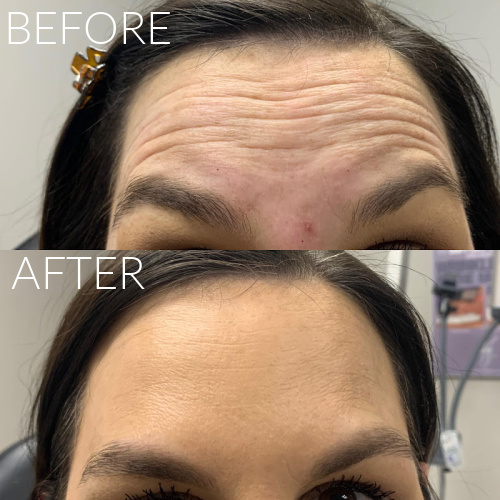
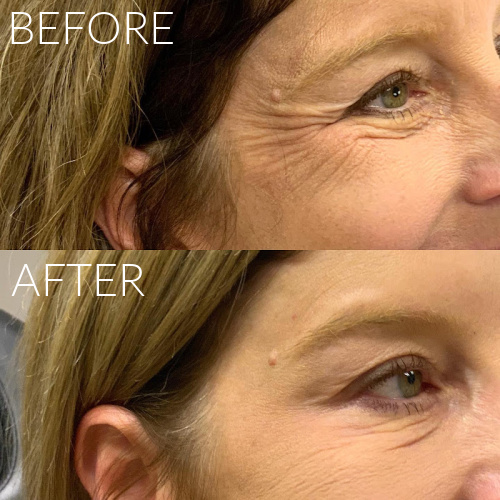
Botox, Dysport, XEOMIN & DAXXIFY
Recommended treatment varies per individual. Results expected to last anywhere from 3-6 months (DAXXIFY may last up to twice as long).
Pricing
Botox | $13/unit |
| Dysport | $5/unit |
| XEOMIN | $13/unit |
| DAXXIFY | $20/unit |
Botox Party Pricing
Botox | $10/unit |
| Dysport | $4.50/unit |
BOTOX FAQ'S
How do I know which Botox treatment is best for me?
During a consultation we will work with you to help you decide which treatment best accomplishes your desired look.
What can you expect after the treatment?
You may begin to notice results within 24 to 48 hours, with full results in 30 days, with results lasting up to four months for moderate to severe frown lines. Remember that results vary from patient to patient. Slight redness, bruising and swelling can be common near the injection areas.
What are the post treatment recommendations?
Avoid rubbing your face and lying down for the first 4 hours after treatment. Minimize strenuous exercise within the first 24 hours following treatment.
How often should I have the treatment?
Recommended treatment varies per individual. Results are expected to last anywhere from 3-6 months.
BOTOX® COSMETIC IMPORTANT INFORMATION
ABOUT BOTOX® COSMETIC?
Choose the brand medical providers and patients know and trust: BOTOX® Cosmetic. It’s the #1 selling treatment of its kind*:
- The first and only treatment FDA-approved to temporarily make moderate to severe frown lines, crow’s feet and forehead lines look better in adults
- Treatment requires minimal downtime. You can return to your daily routine immediately after you leave your specialist’s office
- You may begin to notice results within 24 to 48 hours for moderate to severe frown lines. Full results in 30 days
- It delivers predictable, subtle results, so you look like you, only with less noticeable facial lines
Ask for BOTOX® Cosmetic by name.
TREATMENT YOU CAN TRUST
BOTOX® Cosmetic is an established brand that delivers consistent results. In a survey, people treated (n=381) with BOTOX® Cosmetic say it:
- Is a brand they trust
- Produces predictable results
- Provides subtle results
When surveyed, 62% of respondents (n=342) say treatment is worth the investment.† In fact, BOTOX® Cosmetic is the only treatment of its kind evaluated by the FDA for safety and efficacy in three treatment areas, as well as for patient satisfaction in frown lines and forehead lines in adults.
DYSPORT® IMPORTANT INFORMATION
WHAT IS DYSPORT®?
Natural-looking. Fast-acting. Long-lasting. Don’t let your frown lines between your eyebrows define you. For adults who want a natural look, Dysport is a natural-looking, fast-acting1-3, long-lasting1,2,4 prescription injection proven to help smooth moderate to severe frown lines between the eyebrows—without changing the look or movement of the rest of your face.
Dysport is approved in 69 countries5 and has 25 years of clinical experience worldwide.6* Find out why 97% of women treated with Dysport say they’d do it again.7†‡
*Includes therapeutic and aesthetic uses.†Subject’s satisfaction and self-perception reported 3 weeks after treatment (n=531).‡Design: Multicenter, prospective, non-interventional observational study conducted in France, Germany, Spain and the United Kingdom (n=533). Subjects were eligible if the investigator had decided to prescribe Dysport according to the labeling. Subjects completed questionnaires at 3 weeks and 4 months after treatment.
How does Dysport work?
One injection. Five points.
Dysport temporarily treats moderate to severe frown lines between the eyebrows by reducing specific muscle activity.
Wrinkles are caused by repeated movements and muscle contractions.7 One injection into each of the 5 points between and above the eyebrows temporarily prevents muscle contractions that cause frown lines.
In other words: natural-looking results that look and feel like you.
Who should use Dysport?
See if it’s right for you.
If you’re under 65 and seeking treatment to temporarily improve the look of wrinkles between the eyebrows, ask your specialist if Dysport is right for you. But before you begin, read below to help you make the safest, most informed decision.
- *Includes therapeutic and aesthetic uses.
- †Subject’s satisfaction and self-perception reported 3 weeks after treatment (n=531).
- ‡Design: Multicenter, prospective, non-interventional observational study conducted in France, Germany, Spain, and the United Kingdom (n=533). Subjects were eligible if the investigator had decided to prescribe Dysport according to the labeling. Subjects completed questionnaires at 3 weeks and 4 months after treatment.
- §A secondary endpoint based on Kaplan-Meier estimates of cumulative rate of time to onset of response. The median time to onset of response was 3 days in GL-1 (Dysport 55/105 [52%], Placebo 3/53 [6%]) and GL-2 (Dysport 36/71 [51%], Placebo 9/71 [13%]), and 2 days in GL-3 (Dysport 110/200 [55%], Placebo 4/100 [4%]).
- ‖GL-1 and GL-3 evaluated subjects for at least 150 days following treatment. Based on a ≥1-Grade GLSS improvement from baseline utilizing data from two double-blinded, randomized, placebo-controlled pivotal studies (GL-1, GL-3) in a post-hoc analysis.
- ¶Users = clinical trial subjects.
- #Subject-reported at 12 months (N=120) after two treatments six months apart in a phase IV, multicenter, prospective study.
- **3 out of 10 FACE-Q questions about psychological function are shown.
DO NOT TAKE DYSPORT IF YOU:
- Are allergic to Dysport or any of the ingredients in Dysport. See the end of the Dysport Medication Guide for a list of ingredients in Dysport
- Are allergic to cow’s milk protein
- Had an allergic reaction to any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA) or Xeomin® (incobotulinumtoxinA)
- Have a skin infection at the planned injection site
DYSPORT MAY NOT BE RIGHT FOR YOU IF:
- You have had surgical changes to your face
- You have very weak muscles in the treatment area
- Your face looks very different from side to side
- The injection site is inflamed
- You have droopy eyelids or sagging eyelid folds
- You have deep facial scars
- You have thick, oily skin
- Your wrinkles can’t be smoothed by spreading them apart
The only way to know for sure if Dysport is right for you is to speak with your healthcare provider. Don’t have one? Find a specialist near you who administers Dysport.
XEOMIN® (incobotulinumtoxinA) IMPORTANT INFORMATION
FDA APPROVED. CLINICALLY PROVEN.
Frown lines form when facial expressions are made as the muscle under the skin contracts. Over time, as your skin ages, these repeated expressions cause lasting frown lines. Neurotoxins, such as Xeomin®, are prescription medications that block the release of chemicals that cause these muscle contractions so frown lines are softened.
STATE-OF-THE-ART MANUFACTURING PROCESS
Xeomin® is made through a unique precision manufacturing process that isolates the therapeutic component of the molecule and removes the complexing/unnecessary proteins that don’t play an active role in treatment. Xeomin® is a uniquely purified neurotoxin. Studies have not been performed to determine whether the presence or absence of complexing/unnecessary proteins has a long-term effect on safety or efficacy.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION & APPROVED USES
BOTOX® Cosmetic may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of BOTOX® Cosmetic :
- Problems swallowing, speaking, or breathing, due to weakening of associated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
- Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, and trouble swallowing.
BOTOX® Cosmetic dosing units are not the same as, or comparable to, any other botulinum toxin product .
There has not been a confirmed serious case of spread of toxin effect when BOTOX® Cosmetic has been used at the recommended dose to treat frown lines, crow’s feet lines, and/or forehead lines.
BOTOX® Cosmetic may cause loss of strength or general muscle weakness, vision problems, or dizziness within hours to weeks of taking BOTOX® Cosmetic. If this happens, do not drive a car, operate machinery, or do other dangerous activities.
Serious and/or immediate allergic reactions have been reported. They include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Get medical help right away if you are wheezing or have asthma symptoms, or if you become dizzy or faint.
Do not receive BOTOX® Cosmetic if you : are allergic to any of the ingredients in BOTOX® Cosmetic (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as Myobloc® (rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin® (incobotulinumtoxinA); have a skin infection at the planned injection site.
Tell your doctor about all your muscle or nerve conditions, such as ALS or Lou Gehrig’s disease, myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including difficulty swallowing and difficulty breathing from typical doses of BOTOX® Cosmetic.
Tell your doctor about all your medical conditions, including: plans to have surgery; had surgery on your face; have trouble raising your eyebrows; drooping eyelids; any other abnormal facial change; are pregnant or plan to become pregnant (it is not known if BOTOX® Cosmetic can harm your unborn baby); are breast-feeding or plan to (it is not known if BOTOX® Cosmetic passes into breast milk).
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using BOTOX® Cosmetic with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received BOTOX® Cosmetic in the past.
Tell your doctor if you have received any other botulinum toxin product in the last 4 months; have received injections of botulinum toxin such as Myobloc®, Dysport®, or Xeomin® in the past (tell your doctor exactly which product you received); have recently received an antibiotic by injection; take muscle relaxants; take an allergy or cold medicine; take a sleep medicine; take aspirin-like products or blood thinners.
Other side effects of BOTOX® Cosmetic include : dry mouth; discomfort or pain at the injection site; tiredness; headache; neck pain; and eye problems: double vision, blurred vision, decreased eyesight, drooping eyelids and eyebrows, swelling of your eyelids and dry eyes.
APPROVED USES
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to temporarily improve the look of moderate to severe forehead lines, crow’s feet lines, and frown lines between the eyebrows in adults.
For more information refer to the Medication Guide or talk with your doctor.
To report a side effect, please call Allergan at 1-800-678-1605.
Please see BOTOX® Cosmetic full Product Information including Boxed Warning and Medication Guide.
Dysport® (abobotulinumtoxinA) is a prescription injection for temporary improvement in the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults less than 65 years of age.
Important Safety Information
What is the most important information you should know about Dysport? Spread of Toxin Effects: In some cases, the effects of Dysport and all botulinum toxin products may affect areas of the body away from the injection site. Symptoms can happen hours to weeks after injection and may include swallowing and breathing problems, loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, or loss of bladder control. Swallowing and breathing problems can be life threatening and there have been reports of death. You are at the highest risk if these problems are pre‐existing before injection.
These effects could make it unsafe for you to drive a car, operate machinery, or do other dangerous activities.
Do not have Dysport treatment if you: are allergic to Dysport or any of its ingredients (see the end of the Medication Guide for a list of ingredients), are allergic to cow’s milk protein, had an allergic reaction to any other botulinum toxin product, such as Myobloc®, Botox®, or Xeomin®, have a skin infection at the planned injection site, under 18 years of age, or are pregnant or breastfeeding.
The dose of Dysport is not the same as the dose of any other botulinum toxin product and cannot be compared to the dose of any other product you may have used.
Tell your doctor about any swallowing or breathing difficulties and all your muscle or nerve conditions such as amyotrophic lateral sclerosis [ALS or Lou Gehrig’s disease], myasthenia gravis, or Lambert‐Eaton syndrome, which may increase the risk of serious side effects including difficulty swallowing and difficulty breathing. Serious allergic reactions have occurred with the use of Dysport. Dry eye has also been reported.
Tell your doctor about all of your medical conditions, including if you have surgical changes to your face, very weak muscles in the treatment area, any abnormal facial change, injection site inflammation, droopy eyelids or sagging eyelid folds, deep facial scars, thick oily skin, wrinkles that can’t be smoothed by spreading them apart, or if you are pregnant or breastfeeding or planning to become pregnant or breastfeed.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal and other natural products. Using Dysport with certain other medicines may cause serious side effects. Do not start any new medicines while taking Dysport without talking to your doctor first.
Especially tell your doctor if you: have received any other botulinum toxin product, such as Myobloc® (rimabotulinumtoxinB), Botox® (onabotulinumtoxinA), or Xeomin® (incobotulinumtoxinA), in the last four months or any in the past (be sure your doctor knows exactly which product you received, have recently received an antibiotic by injection, take muscle relaxants, take an allergy or cold medicine, or take a sleep medicine.
Common Side Effects
The most common side effects are nose and throat irritation, headache, injection site pain, injection site skin reaction, upper respiratory tract infection, eyelid swelling, eyelid drooping, sinus inflammation, and nausea.
Ask your doctor if Dysport is right for you.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see Dysport Full Prescribing Information including Medication Guide at DysportUSA.com.
XEOMIN® (incobotulinumtoxinA) IMPORTANT CONSUMER SAFETY INFORMATION
Read the Medication Guide before you start receiving XEOMIN® (Zeo-min) and each time XEOMIN® is given to you as there may be new information. The risk information provided here is not comprehensive. To learn more:
- Talk to your health care provider or pharmacist
- Visit www.xeominaesthetic.com to obtain the FDA-approved product labeling
- Call 1-866-862-1211
Uses XEOMIN® is a prescription medicine that is injected into muscles and used to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults for a short period of time (temporary).
It is not known if XEOMIN is safe and effective in children under 18 years of age.
Warnings
XEOMIN® may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems anytime (hours to weeks) after treatment with XEOMIN®:
- Problems with swallowing, speaking, or breathing can happen within hours to weeks after an injection of XEOMIN® if the muscles that you use to breathe and swallow become weak. Death can happen as a complication if you have severe problems with swallowing or breathing after treatment with XEOMIN®.
- People with certain breathing problems may need to use muscles in their neck to help them breathe and may be at greater risk for serious breathing problems with XEOMIN®.
- Swallowing problems may last for several months, and during that time you may need a feeding tube to receive food and water. If swallowing problems are severe, food or liquids may go into your lungs. People who already have swallowing or breathing problems before receiving XEOMIN® have the highest risk of getting these problems.
- Spread of toxin effects. In some cases, the effect of botulinum toxin may affect areas of the body away from the injection site and cause symptoms of a serious condition called botulism. The symptoms of botulism include: loss of strength and muscle weakness all over the body, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing.
These symptoms can happen hours to weeks after you receive an injection of XEOMIN®. These problems could make it unsafe for you to drive a car or do other dangerous activities.
Do not use XEOMIN® if you are allergic to XEOMIN® or any of the ingredients in XEOMIN® (see the end of this Guide for a list of ingredients in XEOMIN®, had an allergic reaction to any other botulinum toxin products such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®, BOTOX® COSMETIC), or abobotulinumtoxinA (DYSPORT®) or have a skin infection at the planned injection site.
Before receiving XEOMIN®, tell your doctor about all of your medical conditions, including if you
- have a disease that affects your muscles and nerves (such as amyotrophic lateral sclerosis [ALS or Lou Gehrig’s disease], myasthenia gravis or Lambert-Eaton syndrome)
- have had any side effect from any other botulinum toxin in the past
- have a breathing problem such as asthma or emphysema
- have a history of swallowing problems or inhaling food or fluid into your lungs (aspiration)
- have bleeding problems
- have drooping eyelids
- have plans to have surgery
- have had surgery on your face
- are pregnant or plan to become pregnant. It is not known if XEOMIN® can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if XEOMIN® passes into breast milk.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Talk to your doctor before you take any new medicines after you receive XEOMIN.
Using XEOMIN with certain other medicines may cause serious side effects Do not start any new medicines until you have told your doctor that you have received XEOMIN in the past.
Especially tell your doctor if you
- have received any other botulinum toxin product in the last four months
- have received injections of botulinum toxin such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®, BOTOX® COSMETIC) and abobotulinumtoxinA (DYSPORT®) in the past. Be sure your doctor knows exactly which product you received. The dose of XEOMIN® may be different from other botulinum toxin products that you have received.
- have recently received an antibiotic by injection
- take muscle relaxants
- take an allergy or cold medicine
- take a sleep medicine
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
Possible Side Effects
XEOMIN® can cause serious side effects that can be life threatening including
Headache was the most common side effect of XEOMIN® for treatment of glabellar lines. Other possible side effects include:
- dry mouth
- discomfort or pain at the injection site
- tiredness
- neck pain
- muscle weakness
- eye problems, including: double vision, blurred vision, drooping eyelids, swelling of your eyelids, and dry eyes. Reduced blinking can also occur. Tell your doctor or get medical help right away if you have eye pain or irritation following treatment.
XEOMIN® may cause other serious side effects including allergic reactions. Symptoms of an allergic reaction to XEOMIN® may include: itching, rash, redness, swelling, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you get wheezing or asthma symptoms, or if you get dizzy or faint. See “Warnings.”
The most common side effect of XEOMIN in people with glabellar lines include:
- headache
These are not all the possible side effects of XEOMIN®. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of XEOMIN®
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or doctor for information about XEOMIN® that is written for health professionals.
Active Ingredient: botulinum toxin type A
Inactive Ingredients: human albumin and sucrose
Please see accompanying XEOMIN® Full Prescribing Information and Medication Guide.
DAXXIFY® FAQ'S IMPORTANT INFORMATION
What is DAXXIFY®?
The active ingredient in DAXXIFY® is a purified protein called botulinum toxin type A.
*At least 50% of patients in clinical studies had no or minor frown lines 6 months after treatment. Between 3% and 5% of patients in clinical studies had no or minor frown lines 9 months after treatment. In other safety studies, between 5% and 17% of patients still had noticeable improvement 9 months later.
Is DAXXIFY® for me?
Is your frown line treatment not lasting as long as you would like? If so, DAXXIFY® could be the solution for you. Discuss your treatment goals with your aesthetic services provider.
Why does DAXXIFY® have a peptide?
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant.
DAXXIFY® is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
How is DAXXIFY® different from other frown line treatments?
DAXXIFY® is the only long-lasting frown line treatment powered by a peptide.
Results last on average 6 months and up to 9 months for some.†
All frown line treatments require a special ingredient to stabilize botulinum toxin A, the protein responsible for helping smooth moderate to severe frown lines. For instance, BOTOX® Cosmetic uses human serum albumin (HSA), a blood product, as its stabilizer. Dysport®, another frown line treatment, uses HSA as a stabilizer and cow’s milk protein as a protectant.
DAXXIFY® is unique because it is the only formulation that uses a novel peptide as a stabilizer and does not contain human or animal byproducts.
At least 50% of patients in clinical studies had no or minor frown lines 6 months after treatment. Between 3% and 5% of patients in clinical studies had no or minor frown lines 9 months after treatment. In other safety studies, between 5% and 17% of patients still had noticeable improvement 9 months later.
Will I need the same number of Units as my current frown line treatment?
In short, no, and here’s why. Units are like fingerprints—they are a proprietary measurement of a treatment’s unique potency.
Because of the differences in how treatments are formulated and tested, you cannot compare or convert Units between them, just like you cannot compare 2 fingerprints.
A clearer way to understand dosing is to look at how many nanograms of active ingredient (botulinum toxin type A) a product has.
Double the Units does not mean double the dose.
DAXXIFY®
Approved frown line dose: 40 Units
Amount of active ingredient: 0.18 nanograms
BOTOX® Cosmetic
Approved frown line dose: 20 Units
Amount of active ingredient: 0.18 nanograms
As you can see, despite the number of Units, DAXXIFY® isn’t 2x the dose of BOTOX® Cosmetic. Talk to your provider about DAXXIFY® and your aesthetic goals.
Does DAXXIFY® work on all skin types?
The safety and efficacy of DAXXIFY® is well established, with more than 20 years of research. In fact, DAXXIFY® has been studied in the largest-ever phase 3 clinical trial conducted for a frown line treatment and included more than 2,800 people across different ages and with different skin types. DAXXIFY® was studied in patients with varying skin types.
Do long-lasting results mean potentially long-lasting side effects?
No, not necessarily. The most common side effects observed in clinical trials occurred within one to two weeks after injection and lasted only a short while. This is consistent with other frown line treatments.
DAXXIFY® is well-studied, with no serious treatment-related side effects seen in large-scale clinical trials.
Is DAXXIFY® safe?
DAXXIFY® was studied in the largest-ever clinical study for a frown line treatment and included more than 2,800 people across different ages and skin types. Here are some things you should know about DAXXIFY®:
- There were no serious treatment-related side effects
- The active ingredient in DAXXIFY® is botulinum toxin type A, an ingredient that has been used in frown line treatments for more than 20 years
- 96% of people treated with DAXXIFY® were satisfied with their results‡
DAXXIFY® may cause serious side effects that can be life threatening. Please talk to your aesthetic provider about possible side effects and consult with the Prescribing Information and Medication Guide.
96% of patients reported they were satisfied on a 7-point scale when evaluated at 4 weeks in clinical studies.
How should I prepare for my DAXXIFY® treatment?
Ask your aesthetic provider for pre-treatment instructions prior to your DAXXIFY® appointment.
A list of common avoidances before treatment include, but are not limited to:
- Retin-A
- Blood thinners
- Muscle relaxants
- Fish or oils rich in omega 3
- Allergy medication
- Topical steroids
- Sleep medications and supplements
- Aspirin
- St. John’s Wort
- Supplements containing Vitamin E
- Ibuprofen
What can I expect with DAXXIFY® treatment? Does it hurt?
DAXXIFY® injections are typically performed with a small needle and people treated with frown line treatments say the injection feels like a pinch.
If you are concerned about discomfort, talk to your injector about options for minimizing pain, such as icing the area or applying a topical numbing cream. Treatment with DAXXIFY® requires minimal downtime, and you can return to your daily routine immediately after your appointment.
There is a possibility that the area around the injection site will swell, turn red, and cause pain or tenderness from the injection itself; however, these side effects are typically very minor and go away after a few hours or days.
How often will I need to get DAXXIFY®?
If you’re like most people, you probably get a frown line treatment around twice a year. But here’s the thing: conventional treatments only last 3–4 months. If you are not able to get treated every 3-4 months, that means you may spend the next 2-3 months of the year with those frown lines staring back at you between treatments. That’s up to 4-6 months of total wear off time per year!
DAXXIFY® is different. With those same 2 treatment visits, you can keep your frown lines smoother for a full year!§
At least 50% of patients in clinical studies had no or minor frown lines 6 months after treatment.
Will I see results quickly?
You may see results with DAXXIFY® as early as the next day after treatment, and typically within two days.
I have an event coming up in two weeks and want to try DAXXIFY® for the first time. Is now the best time to get DAXXIFY®?
You may see results with DAXXIFY® as early as the next day after treatment, and typically within two days. You should see noticeable results within a two-week time frame. You may experience mild injection-related bruising. In most cases, bruising heals within a few days after injection.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION for DAXXIFY® (daxibotulinumtoxinA-Ianm) injection
DAXXIFY® may cause serious side effects that can be life threatening. Get medical help right away if you have any of these problems any time (hours to weeks) after injection of DAXXIFY®:
• Problems swallowing, speaking, or breathing due to weakening of associated muscles can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
• Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms that include loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice, trouble saying words clearly, loss of bladder control, trouble breathing, and trouble swallowing.
Do not receive DAXXIFY® if you are allergic to any of the ingredients in DAXXIFY® (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as rimabotulinumtoxinB (MYOBLOC®), onabotulinumtoxinA (BOTOX®/BOTOX® Cosmetic), abobotulinumtoxinA (DYSPORT®), incobotulinumtoxinA (XEOMIN®) or prabotulinumtoxinA-xvfs (JEUVEAU®); or have a skin infection at the planned injection site.
DAXXIFY® dosing units are not the same as, or comparable to, any other botulinum toxin product.
Tell your healthcare provider about all your medical conditions, including any side effects from botulinum toxin products, including dry eye; breathing, swallowing, bleeding, or heart problems; plans to have surgery; weakness of forehead muscles; drooping eyelids; have had surgery on your face; are pregnant or breastfeeding or plan to become pregnant or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using DAXXIFY® with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your healthcare provider that you have received DAXXIFY® in the past.
Especially tell your healthcare provider if you have received any other botulinum toxin product in the last 4 months or any in the past, and exactly which product you received (such as BOTOX®, BOTOX® Cosmetic, MYOBLOC®, DYSPORT®, XEOMIN®, or JEUVEAU®). DAXXIFY® may cause serious side effects, including allergic reactions (such as itching, rash, redness, swelling, wheezing, trouble breathing, or dizziness or feeling faint), heart problems (such as irregular heartbeat and heart attack), and eye problems (including dry eye, reduced blinking, and corneal problems). Tell your healthcare provider or get medical help right away if you experience a serious side effect. No serious adverse events of distant spread of toxin effect associated with dermatologic use of DAXXIFY® have been reported in clinical studies at the dose of 40 Units for glabellar lines. The most common side effects of DAXXIFY® include headache, eyelid drooping, and loss of ability to move the muscles in your face.
These are not all the possible side effects of DAXXIFY®. For more information, see the full Prescribing Information including Boxed Warning, and refer to the Medication Guide or talk with your doctor. To report side effects associated with DAXXIFY®, please call 1-877-373-8669. You may also report side effects to the FDA at 1-800-FDA-1088 or visit www.fda.gov/medwatch.
APPROVED USE DAXXIFY® is a prescription medicine that is injected into muscles and used in adults to temporarily improve the look of moderate to severe frown lines between the eyebrows.
DAXI-001723.2