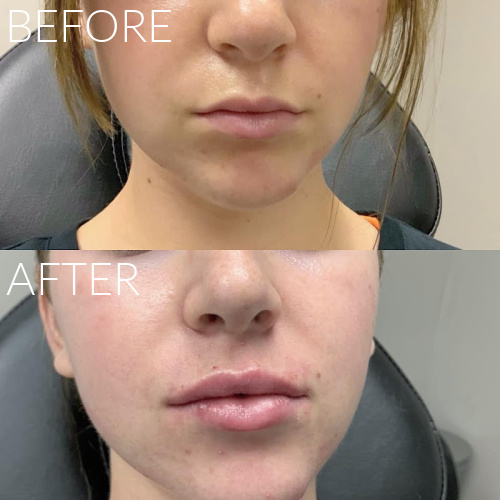
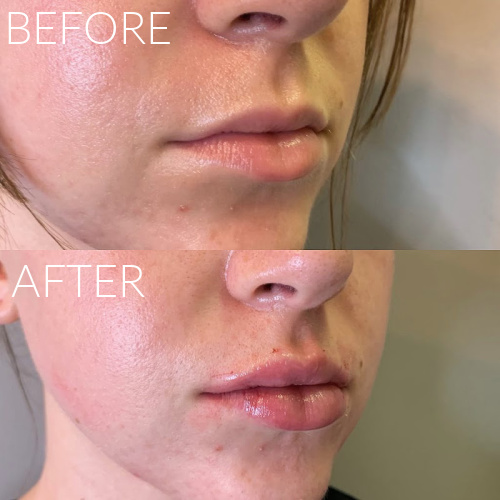
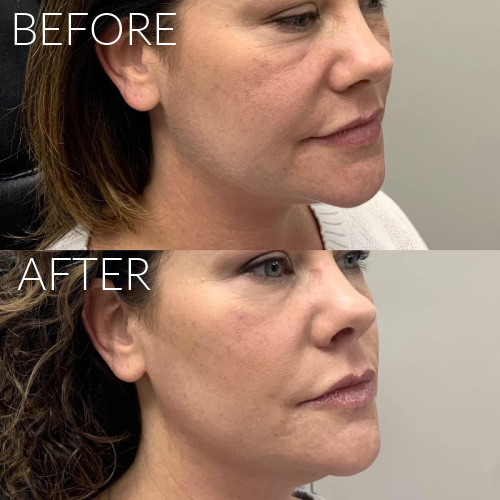
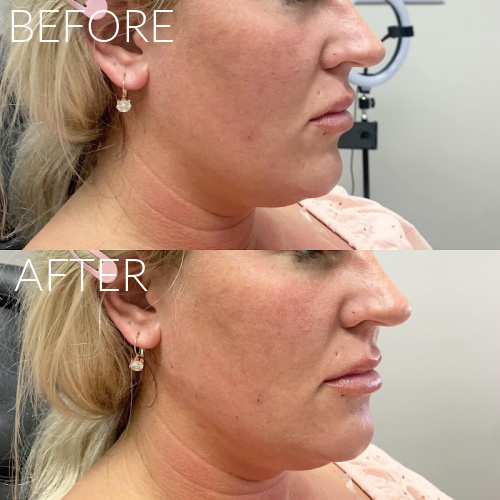
Dermal Fillers
We offer a range of FDA approved facial fillers including Juvederm, Restylane, RHA Collection, Radiesse and Juvederm Voluma. Injectable dermal fillers can plump lips, remove wrinkles, fill in facial creases such as the laugh lines, sculpt the chin and jawline, chin augmentation and fill cheek depressions. Most of our fillers consist of hyaluronic acid which is a natural substance found in your body. We will create a treatment plan with you to give you natural looking results.
Expected results typically last up to one year; Lip Fillers last up to 9 months.
Pricing
Juvederm | $650/syringe |
| Restylane | $650/syringe |
| RHA | /syringe |
| Restylane Contour | $800/syringe |
| Juvederm Voluma | $800/syringe |
DERMAL FILLER FAQ'S
How do I know which filler is best for me?
During a consultation we will work with you to help you decide which treatment best accomplishes your desired look.
What can you expect after the treatment?
Common side effects from filler include bruising, swelling, redness, or tenderness around the injection site, all of which will resolve after a few days to a week.
What are the pre-treament recommendations?
Before getting fillers you should stop using products that contain Retinol, Glycolic Acid or any anit-aging products 2 days before your treatment.
What are the post treatment recommendations?
Minimize strenuous exercise and exposure to extensive sun or heat within the first 24 hours following treatment. Exposure to any of these may cause temporary redness, swelling, and/or itching at the injection site.
How often should I have the treatment?
Results vary per individual. Expected results of filler typically last up to on year. Lip fillers last up to 9 months.
JUVÉDERM® INJECTABLE GEL FILLERS IMPORTANT INFORMATION
APPROVED USES
JUVÉDERM® VOLUMA™ XC injectable gel is for deep injection in the cheek area to correct age-related volume loss and for augmentation of the chin region to improve the chin profile in adults over 21.
JUVÉDERM® VOLLURE™ XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC injectable gels are for injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. JUVÉDERM® VOLLURE™ XC injectable gel is for adults over 21.
JUVÉDERM® VOLBELLA™ XC injectable gel is for injection into the lips for lip augmentation and for correction of perioral lines in adults over 21.
JUVÉDERM® Ultra XC injectable gel is for injection into the lips and perioral area for lip augmentation in adults over 21.
RESTYLANE® IMPORTANT INFORMATION
The Restylane® line smooths away facial wrinkles and folds ( Restylane,5 Restylane Lyft,6 Restylane Refyne,7 Restylane Defyne8 ), provides subtle lip enhancement (Restylane,5 Restylane Silk9), smooths the lines around the mouth (Restylane Silk9), adds lift and volume to the cheeks (Restylane Lyft6), and helps reverse the signs of volume loss in the back of the hands (Restylane Lyft6).
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive any JUVÉDERM® formulation?
Do not use these products if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), or if you are allergic to lidocaine or the Gram-positive bacterial proteins used in these products.
What precautions should my doctor advise me about?
- Minimize strenuous exercise and exposure to extensive sun or heat within the first 24 hours following treatment. Exposure to any of these may cause temporary redness, swelling, and/or itching at the injection site
- Tell your doctor if you are pregnant or breastfeeding. The safety of these products for use during pregnancy or while breastfeeding has not been studied
- The safety of JUVÉDERM® VOLUMA™ XC has not been studied in patients under 35 years or over 65 years for cheek augmentation, or under 22 years and over 80 years for chin augmentation. The safety of JUVÉDERM® VOLLURE™ XC and JUVÉDERM® VOLBELLA™ XC has not been studied in patients under 22 years, and the safety of JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC has not been studied in patients under 18 years
- JUVÉDERM® VOLUMA™ XC is intended for use in the chin and cheek areas. JUVÉDERM® VOLLURE™ XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC are intended for use in facial wrinkles and folds. JUVÉDERM® VOLBELLA™ XC and JUVÉDERM® Ultra XC are intended for use in the lips and perioral area. The safety and effectiveness for treatment in other areas have not been established in clinical studies
- Tell your doctor if you have a history of excessive scarring (thick, hard scars) or pigmentation disorders. The safety of JUVÉDERM® products has not been studied in these patients and may result in additional scars or changes in pigmentation
- Tell your doctor if you are on therapy used to decrease the body’s immune response (immunosuppressive therapy). Use may result in an increased risk of infection
- Tell your doctor before treatment if you are using substances that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners. As with any injection, this may result in increased bruising or bleeding at the injection site
- Patients who experience skin injury near the site of injection may be at a higher risk for adverse events
- JUVÉDERM® VOLUMA™ XC was not studied in patients with significant loose skin of the chin, neck, or jaw
- The effect of JUVÉDERM® VOLUMA™ XC injection into the chin on facial hair growth has not been studied
What are possible side effects?
The most commonly reported side effects with JUVÉDERM® injectable gels included redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA™ XC, dryness was also reported. For JUVÉDERM® VOLUMA™ XC, most side effects resolved within 2 to 4 weeks. For JUVÉDERM® VOLLURE™ XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC injectable gels, most resolved within 14 days or less. For JUVÉDERM® VOLBELLA™ XC, most resolved within 30 days or less. These side effects are consistent with other facial injection procedures.
Most side effects will resolve with time. Your doctor may choose to treat side effects persisting over 30 days with antibiotics, steroids, or hyaluronidase (an enzyme that breaks down hyaluronic acid).
One of the risks with these products is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin.
As with all skin injection procedures, there is a risk of infection.
Visit Juvederm.com or talk to your doctor for more information. To report a side effect with any JUVÉDERM® product, please call Allergan at 1-800-433-8871.
Products in the JUVÉDERM® Collection are available only by a licensed physician or properly licensed practitioner.
RADIESSE® and RADIESSE® (+) IMPORTANT CONSUMER SAFETY INFORMATION
Who should not use RADIESSE® or RADIESSE® (+)?
You should not use RADIESSE® or RADIESSE® (+) if you have an allergy to any component of the product, if you have a history of severe allergies, if you have a bleeding disorder, or if you are pregnant or breastfeeding. You should not use RADIESSE® (+) if you have an allergy to lidocaine or medicines like it.
What is the most important information I should know about RADIESSE® and RADIESSE® (+)?
One of the risks with using these products is unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious, and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms, or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin, or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
As with all procedures that involve an injection through the skin, there is a risk of infection.
Do not use RADIESSE® or RADIESSE® (+) if you have a skin infection until it has healed.
It is not known if RADIESSE® or RADIESSE® (+) is safe or effective in the lips, or in the area around the eyes.
The microspheres in RADIESSE® and RADIESSE® (+) can be seen in X-rays and CT Scans. It is very important that you tell your health care provider that you have had RADIESSE® or RADIESSE® (+) dermal filler.
If you have a history of herpes, you may experience a herpes breakout after receiving RADIESSE® or RADIESSE® (+).
Injection in the back of the hand may result in temporary difficulty performing activities. RADIESSE® may cause nodules, bumps or lumps in the back of the hand and can last up to 1 year.
You should minimize strenuous activity and avoid extensive sun or heat exposure for about 24 hours after treatment and until any swelling or redness has resolved.
What should I tell my doctor before using RADIESSE® or RADIESSE® (+)?
Tell your health care provider if you are taking blood thinners or medicines that can interfere with the clotting of blood, such as aspirin or warfarin. These medicines might make it more likely that you will experience bruising or bleeding at the injection site. Tell your health care provider if you have any diseases, injuries or disabilities of the hand, if you have a history forming large, raised scars or if you have had any other skin treatments such as skin peels.
What are the most common adverse events with RADIESSE® or RADIESSE® (+)?
The most common adverse events seen in clinical studies of RADIESSE® used in the hands include bruising, redness, swelling, pain, itching, nodules or bumps/lumps, difficulty performing activities, loss of sensation and other local side effects. The most common adverse events seen in clinical studies of RADIESSE® or RADIESSE® (+) used in the face include bruising, redness, swelling, pain, itching and other local side effects.
These are not all of the possible side effects with RADIESSE® or RADIESSE® (+). Merz collects information about adverse events seen with RADIESSE® and RADIESSE® (+) outside of clinical studies. These events are included in the RADIESSE® and RADIESSE® (+) Patient Information Guide based on an assessment of seriousness and potential causal relationship to RADIESSE® or RADIESSE® (+).
Please see the Patient Information Guide available at www.radiesse.com for list of these events. Tell your health care provider about any side effects that bother you or do not go away.
Important: For full safety information, please visit www.Radiesse.com or call MyMerz Solutions at 1-844-469-6379
RADIESSE® and RADIESSE® (+) are available by prescription only.
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Kysse, Restylane® Refyne, Restylane® Defyne, and Restylane® Contour.
APPROVED USES
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds. Restylane® Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild to moderate chin retrusion.
Restylane® Contour is for cheek augmentation and for the correction of midface contour deficiencies.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To report a side effect with any Restylane® product, please call Galderma Laboratories, L.P at 1-855-425-8722.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
RHA® Collection Important Information
The RHA® Collection is Resilient Hyaluronic Acid (HA) dermal fillers designed for facial dynamics. The collection represents the latest advancement in HA technology in more than a decade. The RHA® Collection uses a gentle manufacturing process to more closely resemble the natural HA in the skin—for clean beauty.
The RHA® Collection offers four HA-filler formulations. RHA Redensity™ is a weightless filler that smooths delicate lipstick lines. RHA® 2 and RHA® 3 offer elegant and refined smoothing for those seeking a soft, natural look in wrinkles and folds. Lastly, RHA® 4 provides natural volume for deeper dynamic wrinkles and folds.
Why is a dynamic filler important?
Areas treated by HA fillers can go back to their original shape after being altered, stretched, bent, or compressed. Fillers need to have a high level of resilience because they are used in dynamic areas of the face, such as around the mouth. With all of the micromovements we make throughout the day like smiling, laughing, and talking, it’s important to have a dynamic filler that can adapt and be resilient to the movements in the face. The RHA® Collection is specifically designed for facial dynamics and a natural look at rest and in motion.
The RHA® Collection is widely studied. The clinical effectiveness and safety of RHA® 2, RHA® 3, and RHA® 4 were shown in two 15-month studies for wrinkles and folds. The clinical effectiveness and safety of RHA Redensity™ were shown in a 12-month study for lipstick lines—in which 2/3 of participants still had lipstick-line improvement at 12 months.
The RHA® Collection is the only line of FDA-approved HA fillers for dynamic lines, wrinkles, and folds.
The RHA® Collection offers four HA-filler formulations. RHA Redensity™ is a weightless filler that smooths delicate lipstick lines. RHA® 2 and RHA® 3 offer elegant and refined smoothing for those seeking a soft, natural look in wrinkles and folds. Lastly, RHA® 4 provides natural volume for deeper dynamic wrinkles and folds.
The RHA® Collection uses a gentle manufacturing process with minimal chemical modifications to the initial composition. This helps preserve the structure of HA, helping it more closely resemble the natural HA found in the skin. The RHA® Collection is the latest advancement in HA-filler technology—purposely designed to treat dynamic areas of the face.
The RHA® Collection was developed by the innovative Swiss company, TEOXANE, and has been widely used by aesthetic experts outside of the US since 2015. TEOXANE was founded by Madame Valerie Taupin who co-created the dermal filler Juvéderm® two decades ago, looking to perfect dermal-filler technology. She then directed her research team to design an HA filler to more closely mimic HA in the skin—leading to the latest filler innovation in the market: RHA® Collection.
IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive any RHA® injectable gel formulation?
Do not receive if you have a history of multiple severe allergies or severe allergic reactions; if you are allergic to lidocaine or gram-positive bacterial proteins; or if you have a bleeding disorder.
What precautions should I discuss with my doctor?
• Tell your doctor if you are pregnant or breastfeeding as the safety of these products for use during pregnancy or while breastfeeding has not been studied
• Tell your doctor if you have a history of excessive scarring, keloid formations or pigmentation disorders, as use of these products may result in additional scars or changes in pigmentation
• Tell your doctor if you are planning laser treatments or a chemical peel, as there is a possible risk of inflammation at the treatment site if these procedures are performed after treatment
• Tell your doctor if you are on immunosuppressive therapy used to decrease your immune response, as use of these products may result in an increased risk of infection
• Tell your doctor if you are using medications that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners, as this may increase bruising or bleeding at the injection site
• The safety and effectiveness of RHA® fillers in areas other than those indicated have not been established in U.S. clinical studies
• Patients who experience skin injury near the site of injection with this product may be at a higher risk for side effects
• Minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment
To report a side effect with any RHA® product, please call Revance at (877) 373-8669. Please visit RHACollection.com or talk to your doctor for more information.
Available by prescription only.